Abstract
Introduction
Oxidative stress and inflammation promote hemolysis and vaso-occlusion in sickle cell disease (SCD). Erythrocytes can play a pro-oxidative and anti-oxidative role in disease-associated inflammation through iron-driven free radical production and endogenous anti-oxidants. In SCD, HbS accelerated auto-oxidation, iron de-compartmentalization, and inflammatory cell-derived oxidants drive this oxidative stress. CD33-related Sialic acid-binding immunoglobulin-type lectins (CD33rSiglecs) are cell surface proteins that recognize sialic acids in "Self-Associated Molecular Patterns" (SAMPs) and typically inhibit innate immune cell functions via cytosolic signaling. Recent studies have shown that Siglec-9 on human neutrophils in circulating blood interact with erythrocyte sialic acids (prominently glycophorin-A (GYPA) to suppress neutrophil reactivity, including reactive oxygen species (ROS) production. Modification of erythrocyte membrane sialic acids interferes with their ability to inhibit neutrophil activation and oxidative burst. As erythrocytes age and undergo cellular damage there is a loss of membrane bound sialoglycoproteins. Several studies have indicated that this reduction in the sialome is accelerated on the sickle erythrocyte. We hypothesize that altered sickle erythrocyte membrane sialic acid leads to decreased Siglec-9 binding capability, and that decreased binding of neutrophil Siglec-9 to sickle erythrocyte sialic acid enhances neutrophil activation and oxidative burst.
Methods & Results
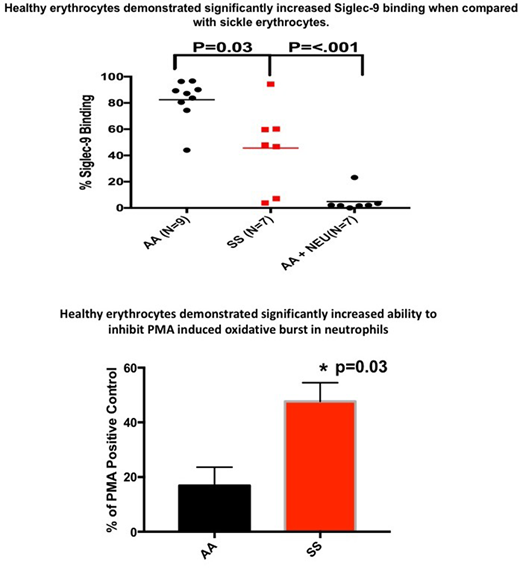
Binding of recombinant Siglec-9-Fc protein to AA (n=9) and SS erythrocytes (n=7) was measured using flow cytometry. SS erythrocytes displayed significantly less Siglec-9-Fc binding 45% ± 11.9 (mean ± SEM) compared to AA erythrocytes 82% ± 5.4 (p=.03). Treatment of AA erythrocytes with neuraminidase to remove sialic acid decreased binding to 4% ± 7.9.
Neutrophil ROS production was measured by flow cytometry using dihydrorhodamine 123 (DHR123). Neutrophils were purified from AA donors (n=5) and SS (n=11) or AA erythrocytes (n=5) were added to the neutrophils at a ratio of 50 erythrocytes to 1 neutrophil. Oxidative burst was stimulated using phorbol 12-myristate 13-acetate (PMA). AA erythrocytes decreased PMA-stimulated neutrophil ROS production by 84% ± 6.7. In contrast, SS erythrocytes decreased PMA-stimulated neutrophil ROS production by 53% ± 6.8 (p=0.03,).
Recent studies have shown that neutrophil extracellular trap (NET) formation is pathogenic in SCD. We added AA and SS erythrocytes (50:1) to neutrophils stimulated with PMA and NET formation was assessed using Syto Orange, Syto Green and confocal microscopy. PMA-stimulated neutrophils incubated with AA erythrocytes showed minimal NET formation. In contrast, AA erythrocytes treated with neuraminidase to remove sialic acid had increased NET formation. PMA-stimulated neutrophils incubated with SS erythrocytes showed increased NET formation.
Conclusions
A constant disease state of oxidative stress and inflammation underlies SCD pathophysiology. These data demonstrate that SS erythrocytes with decreased membrane sialic acid are deficient in binding to neutrophil Siglec-9. The decreased binding of SS erythrocytes to neutrophil Siglec-9 diminishes the ability of SS erythrocytes to properly modulate neutrophil activation, which may contribute to the oxidative stress and increased state of basal inflammation inherent to SCD. The overall reduction in number of RBCs in SS may also be a factor?
Belcher:CSL Behring: Research Funding. Vercellotti:CSL Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal